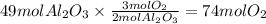Answer:
2.6 × 10³ g Al
74 mol O₂
Step-by-step explanation:
Aluminum oxide decomposes into aluminum and oxygen, according to the following balanced equation.
2 Al₂O₃ ⇒ 4 Al + 3 O₂
Step 1: Calculate the moles corresponding to 5.0 kg of aluminum oxide
The molar mass of aluminum oxide is 101.96 g/mol.
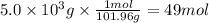
Step 2: Calculate the mass of aluminum formed
We will use the following relations.
- The molar ratio of Al₂O₃ to Al is 2:4
- The molar mass of Al is 26.98 g/mol
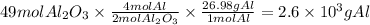
Step 3: Calculate the moles of oxygen produced
The molar ratio of Al₂O₃ to O₂ is 2:3. Then,