Answer:
19.47%
Step-by-step explanation:
The percentage by mass of
 would be 19.47%.
would be 19.47%.
The percentage by mass of a substance in solution is calculated as:
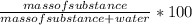
Given that the mole fraction of
 = 0.432
= 0.432
The total number of mole fraction of a solution = 1
Hence, mole fraction of water = 1 - 0.432 = 0.568
Mass of water = molar mass x mole
= 98.079 x 0.432 = 42.37 g
Mass of
 = molar mass x mole
= molar mass x mole
= 18.02 x 0.568 = 10.24 g
Hence,
%mass of
 =
=
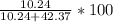
= 19.47%