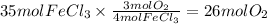Answer:
26 mol
Step-by-step explanation:
Step 1: Write the balanced equation
4 FeCl₃ + 3 O₂ ⇒ 2 Fe₂O₃ + 3 Cl₂
Step 2: Determine the appropriate molar ratio
The molar ratio of FeCl₃ to O₂ is 4:3.
Step 3: Use the determined molar ratio to calculate the moles of oxygen required to completely react with 35 moles of ferric chloride