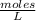Answer:
The molarity of a 50.0 ml aqueous solution containing 10.0 grams of table sal, Nacl, is 3.42
Step-by-step explanation:
Molarity is a unit of concentration based on the volume of a solution and is defined as the number of moles of solute per liter of solution. Then, the molarity of a solution is calculated by dividing the moles of the solute by the liters of the solution.
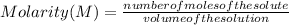
Molarity is expressed in units (
 ).
).
Then you must know the amount of moles of the NaCl solute. For that it is necessary to know the molar mass. Being:
- Na: 23 g/mole
- Cl: 35.45 g/mole
the molar mass of NaCl is: 23 g/mole + 35.45 g/mole= 58.45 g/mole
Then a rule of three applies as follows: if 58.45 grams are present in 1 mole of NaCl, 10 grams in how many moles will they be?
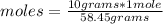
moles= 0.171
So you know:
- number of moles of solute= 0.171 moles
- volume= 50 mL= 0.05 L
Replacing in the definition of molarity:
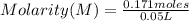
Solving:
Molarity= 3.42
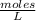
The molarity of a 50.0 ml aqueous solution containing 10.0 grams of table sal, Nacl, is 3.42