Answer:
Limiting reactant is CS2
% yield = 131.8 %
Step-by-step explanation:
Using the alanced equation given, Mole ratio of C to CS2 is 1 : 5.
That is, for every 1 mole of CS2 used, 5 moles of Carbon is produced.
35g of CS2 ---
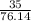 moles
moles
= 0.4597 moles
⇒ mass of C produced = 5 × 0.4597 × 12 g/mol
= 27.6 g of C.
Percentage yield (%) =
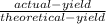
=
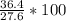 =131.9%
=131.9%