Answer:
The minimum energy required to remove an electron from potassium metal is 3.465 x 10⁻¹⁹ J
Step-by-step explanation:
Given;
light wavelength, λ = 570nm
velocity of ejected electron, v = 6.4 x 10⁴ m/s
Minimum energy required to remove an electron from a metal surface is given as;
W₀ = hf₀
where;
W₀ is work function also known as minimum energy required
h is Planck's constant
f₀ is threshold frequency of light
Also,
E = W₀ + K.E
where;
E is the energy of the incident light
W₀ is work function
K.E is the kinetic energy of the electron
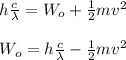
where;
m is mass of electron
c is speed of light
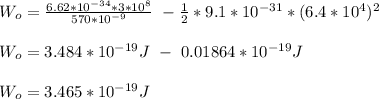
Therefore, the minimum energy required to remove an electron from potassium metal is 3.465 x 10⁻¹⁹ J