Answer:
A. 5.6 10⁻⁹M
Step-by-step explanation:
Hello,
In this case, we should know that the pH it related with the pOH by:
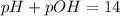
This allows us to compute the pOH and subsequently the concentration of hydroxyl ions, [OH-], as shown below:
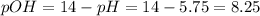
Thus, since pOH is mathematically defined as:
![pOH=-log([OH^-])](https://img.qammunity.org/2021/formulas/chemistry/college/os6i8jhsq2yoevim3ewlj1jvyoxoba6pi1.png)
We finally compute [OH-] as follows:
![[OH^-]=10^(-pOH)=10^(-8.25)\\\\](https://img.qammunity.org/2021/formulas/chemistry/college/kq8gc8ew3wm9bt4hzfpoiw77iskddl5kz7.png)
![[OH^-]=5.6x10^(-9)M](https://img.qammunity.org/2021/formulas/chemistry/college/ucojykftyvdbi2ktub94kf6f1697sggxxu.png)
Hence, for sure the answer is A. 5.6 10⁻⁹M
Best regards.