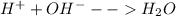Answer:
Ca(OH)2 (aq)
Step-by-step explanation:
The base in the reaction would be Ca(OH)2 (aq).
A base is a substance which when dissolved in water, releases
 as the only negative ion. A base is also a substance that donates electrons and accept protons in a reaction.
as the only negative ion. A base is also a substance that donates electrons and accept protons in a reaction.
Ca(OH)2 in aqueous solution will ionize thus:
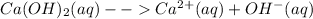
In a reaction involving an acid, the hydroxyl ion donates its electron and accept proton.