Answer:
Change in energy of the gas mixture during the reaction is 32.0 kJ.
Step-by-step explanation:
For a reaction occurring at constant pressure (P)-
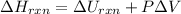
where
 ,
,
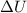 and
and
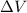 represent change in enthalpy, change in internal energy and change in volume respectively.
represent change in enthalpy, change in internal energy and change in volume respectively.
 represents work done at constant pressure.
represents work done at constant pressure.
Here change in energy of the gas mixture actually indicates change in internal energy of the gaseous mixture.
Here
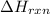 = -162. kJ,
= -162. kJ,
 = -194. kJ
= -194. kJ
So
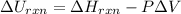
= (-162. kJ) - (-194. kJ)
= 32.0 kJ