Answer:
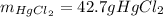
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
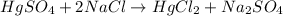
In such a way, the mercury II sulfate (molar mass 296.65g/mol) is in a 1:1 molar ratio with the mercury II chloride (molar mass 271.52g/mol), for that reason the stoichiometry to find mass in grams of mercury II chloride turns out:
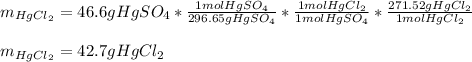
Best regards.