Answer:
4.352 grams of oxygen will be consumed.
Step-by-step explanation:
First of all you should bear in mind that the balanced chemical equation is:
4 Cu + O₂ → 2 Cu₂O
Then you can apply a rule of three as follows: if 4 moles of copper react with 1 mole of oxygen by stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), 0.544 moles of copper with how many moles of oxygen do they react?
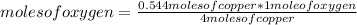
moles of oxygen= 0.136
On the other hand, the molar mass of oxygen O2 is 32 g / mol (the mass of an oxygen being 16 g / mol). Then the mass that will be consumed is calculated using a rule of three: if 1 mole of oxygen has 32 g, 0.136 moles how much mass does it have?
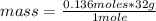
mass= 4.352 g
4.352 grams of oxygen will be consumed.