Ok I am not so sure about this one because I am new to learning the combined gas law but I think this is how you do it. So to solve this I would use the combined gas law which is:
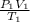 =
=
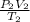
The 1 and 2 are the variable before and after they are compressed
P is the pressure in atm
V is the volume in L
T is the temperature in Kelvin
So first you need you to plug in all of the numbers in the variables.
Side note: standard temp is 273 Kelvin and standard pressure is 1 atm, also I converted the mL to L by dividing by 1000
(1)(4)/(divided by)/273K = P(0.346L)/(divided by)/273K then simplified its:
0.015 = 0.0013P then divide both sides by 0.0013
Which makes P=11.54
( I rounded most of my answers so it could be a bit different )