Answer:
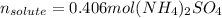
Step-by-step explanation:
Hello,
In this case, since the molarity is defined as the ratio of the moles of the solute to the volume of the solution in liters:
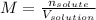
For the given volume and molarity, we solve for the moles of ammonium sulfate (solute) as shown below:
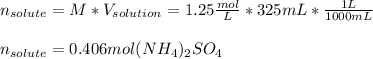
Best regards.