Answer:
1 mole of Sn is required to react with 40 gram of HF
Step-by-step explanation:
It is given that molar mass of HF is 20.01 g/mol
Mass of HF is 40 gram
So number of of HF is
Number of moles
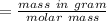
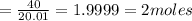
When reaction between HF and Sn takes place
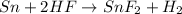
From the reaction it is clear that 1 mole Sn require 2 moles HF
Therefore 2 moles of HF require 1 mole Sn