Answer:
New volume of the gas will be 9.33 L.
Step-by-step explanation:
Let's assume the gas behaves ideally.
So we can write,
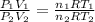
where
 and
and
 represents initial and final pressure of gas respectively.
represents initial and final pressure of gas respectively.
 and
and
 represents initial and final volume of gas respectively
represents initial and final volume of gas respectively
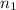 and
and
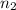 represents initial and final number of moles of gas respectively.
represents initial and final number of moles of gas respectively.
 and
and
 represents initial and final temperature (in kelvin scale) of gas respectively.
represents initial and final temperature (in kelvin scale) of gas respectively.
R is gas constant.
Here
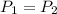 ,
,
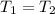 ,
,
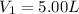 ,
,
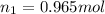 and
and
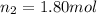
So
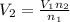 =
=
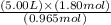 = 9.33 L
= 9.33 L
Hence new volume of the gas will be 9.33 L.