Answer:
Mass concentration (g/L) = 2.49g/L.
Step-by-step explanation:
No. of moles =

=
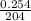 = 0.001245 moles
= 0.001245 moles
Concentration of KHP (C1) in litres = n/v
=
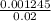 = 0.062 mol/L
= 0.062 mol/L
We know that:
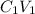 =
=
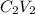
where c1v1 and c2v2 are the products of concentration and volumes of KHP and NaOH respectively.
Since mole ratio is 1 : 1.
1 mole of NaOH - 40g
0.001245 mole of NaOH = 40 × 0.001245 = 0.0498g
⇒0.0498g of NaOH was used during the titration
∴Mass concentration (g/L) = 0.0498g ÷ 0.02L
= 2.49g/L.