Answer:
ΔG = -139kJ/mol
Step-by-step explanation:
The formula to find ΔG in a non-standard state is:
ΔG = ΔG° + RT ln Q
Where ΔG° is ΔG value for standard state, R is gas constant (0.008314 kJ/molK); T is absolute temperature (298K); And Q is reaction quotient.
For the reaction:
NO(g) + O₃(g) ⇄ NO₂(g) + O₂(g)
Reaction quotient, Q, is:
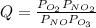
Where P represents the pressures in the non-standard state
Replacing:
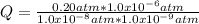
Q = 2.0x10¹⁰
ΔG = -198kJ/mol + 0.008314kJ/molK×298K ln 2.0x10¹⁰
-You can use also 8.314kJ/molK as constant but it is necessary the consistency in units-
ΔG = -139kJ/mol