Answer:
Mole fraction of
 = 0.42
= 0.42
Moles fraction of Ar = 0.037
Step-by-step explanation:
Let's assume all the four gases and their mixture behave ideally.
According to Dalton's law of partial pressure for mixture of ideal gases-
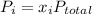
where
 and
and
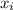 are partial pressure and mole fraction of "i"-th gas respectively.
are partial pressure and mole fraction of "i"-th gas respectively.
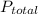 is total pressure of gaseous mixture.
is total pressure of gaseous mixture.
Here
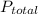 =
=

= (3.5+2.8+0.25+0.15) atm
= 6.7 atm
So
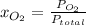 =
=
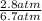 = 0.42
= 0.42
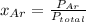 =
=
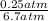 = 0.037
= 0.037