We have been given that element X decays radioactively with a half life of 13 minutes. There are 260 grams of Element X. We are asked to find the minutes it will take the element to decay to 15 grams.
We will use half-life formula to solve our given problem.
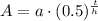 , where,
, where,
A = Final amount,
a = Initial amount,
t = Time,
h = Half life.
We have
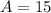 ,
,
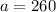 ,
,
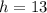 .
.
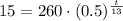
Let us solve for t.
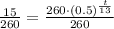
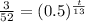
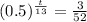
Now we will take natural log on both sides.
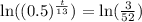
Using natural log property
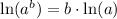 , we will get:
, we will get:
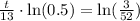
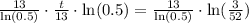
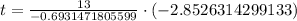
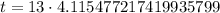
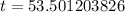
Upon rounding to nearest tenth of minute, we will get:
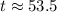
Therefore, it will take approximately 53.5 minutes for the element to decay to 15 grams.