Answer:
Silicon is the limiting reactant and nitrogen the excess reactant.
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
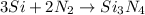
Thus, to identify the limiting reactant, we shall compute the available moles of silicon and the moles of silicon that will be consumed by the 500 g of gaseous nitrogen, considering their 3:2 molar ratio in the chemical reaction, as shown below:
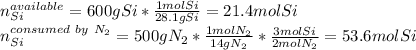
Hence, we notice that 500 g of nitrogen will consume 53.6 moles of silicon but 21.4 moles are available only, for that reason, silicon is the limiting reactant and nitrogen the excess reactant.
Best regards.