Answer:
3.91x10²³ atoms of lead
Step-by-step explanation:
In chemistry, a mole of a substance is defined as 6.022x10²³ particles that could be atoms, molecules, ions, etc.
As you can see, in the problem, you have 0.650moles of lead in a fishing line sinker, the present atoms are:
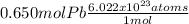 = 3.91x10²³ atoms of lead
= 3.91x10²³ atoms of lead