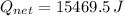Answer:
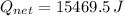
Step-by-step explanation:
Let assume that liquid water has an initial temperature of 25°C and heating and evaporation process occurs under a pressure of 1 atmosphere. Then, the heat absorbed is equal to the sum of sensible and latent components:
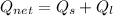
![Q_(net) = (6\,g)\cdot \left[\left(4.186\,(J)/(g\cdot ^(\circ)C)\right)\cdot (100\,^(\circ)C-25\,^(\circ)C)+2264.3\,(J)/(g) \right]](https://img.qammunity.org/2021/formulas/chemistry/high-school/monmapy3pfd0cvtpjbjae5phbgs9n7zq2t.png)