Answer:
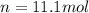
Step-by-step explanation:
Hello,
In this case, we use the ideal gas equation which allows us to understand the pressure-volume-mole-temperature behavior of hydrogen gas:

Thus, solving for the moles, we obtain:
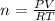
We cannot forget that the temperature should be used in absolute units (Kelvins), therefore, the resulting moles turn out:
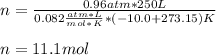
Best regards.