Answer:
![[HCl]_(eq)=0.05M](https://img.qammunity.org/2021/formulas/chemistry/college/7llvj6da3p6z9dgvwb2ecoa5t6ezpb616r.png)
Step-by-step explanation:
Hello,
In this case, for the given chemical reaction, the law of mass action at equilibrium results:
![Kc=([H_2]_(eq)[Cl_2]_(eq))/([HCl]^2_(eq))](https://img.qammunity.org/2021/formulas/chemistry/college/3t09oo7ypo1hx24rn4rf7du0og7o3r40un.png)
Next, in terms of the change
 due to reaction extent, it is rewritten, considering an initial concentration of HCl of 0.25M (1mol/4L), as:
due to reaction extent, it is rewritten, considering an initial concentration of HCl of 0.25M (1mol/4L), as:
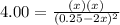
Thus, solving for
 via quadratic equation or solver, the following results are obtained:
via quadratic equation or solver, the following results are obtained:
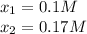
Clearly, the solution is
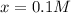 as the other result will provide a negative concentration for the hydrochloric acid at equilibrium, thereby, its equilibrium concentration turns out:
as the other result will provide a negative concentration for the hydrochloric acid at equilibrium, thereby, its equilibrium concentration turns out:
![[HCl]_(eq)=0.25M-2*0.1M](https://img.qammunity.org/2021/formulas/chemistry/college/uamlmqlx3830lx0z2ax0jz4pqt1jdivb7a.png)
![[HCl]_(eq)=0.05M](https://img.qammunity.org/2021/formulas/chemistry/college/7llvj6da3p6z9dgvwb2ecoa5t6ezpb616r.png)
Best regards.