Answer:
The accurate label for the solution is 2.00 mol/L NaCl.
Step-by-step explanation:
Molrity = mass ÷ molar mass
Molar mass of NaCL = 58.44 g/mol
Weighed mass = 116.9g
⇒ Molarity of the solution =
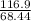 = 2.0M
= 2.0M
Therefore, the accurate label for the solution is 2.00 mol/L NaCl.