Answer:
The final energy of the system is 168 J
Step-by-step explanation:
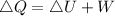
Heat energy released to the surroundings,
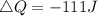
Work done, W = 222 J
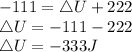
The initial internal energy,
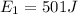
The change in internal energy is given by the formula,
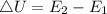
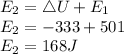
The final energy of the system is 168 J