Answer:
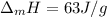
Or:
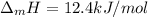
Step-by-step explanation:
Hello,
In this case, since the required heat to turn solid gold into liquid gold is computed by:
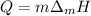
Which is mass and enthalpy of fusion- dependent, we compute the enthalpy of fusion as shown below:
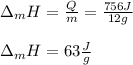
Or in kJ/mol (typical data):
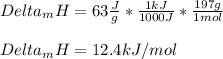
Best regards.