Answer:
2πc/w
Step-by-step explanation:
To find the wavelength you take into account the difference in energy of two adjacent states n+1 and n:
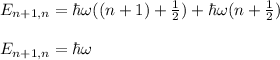 (1)
(1)
hbar = h/2π
this energy is also the energy of an emitted photon in the transition, that is:
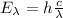 (2)
(2)
you equal the equations (1) and (2) and compute the wavelength:
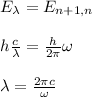
hence, the wavelength of the emitted photon is 2πc/w