Answer: 22g of chlorine would be needed to carry out this synthesis reaction
Step-by-step explanation:
A synthesis reaction is one in which two or more than two elements combine together to forma single product.
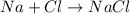
The atoms present in the reactants are found on the product side. According to the law of conservation of mass, the number of atoms on both sides of the arrow must be same as the total mass must be conserved.
15 grams of sodium reacts with 22 grams of chlorine to yield 37 grams of sodium chloride. Thus 22g of chlorine would be needed to carry out this synthesis reaction.