Answer:
1.00 (%w/v)
Step-by-step explanation:
%w/v is an unit of concentration in chemistry defined as the ratio between mass of the solute in grams and volume of solvent in mL times 100.
In the problem, the solute is D-mannitol -Less quantity-. The mass of the solute is 10g
The volume of the solvent is 1L = 1000mL
Thus, %w/v is:
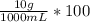 = 1.00 (%w/v)
= 1.00 (%w/v)