Answer:
195mmHg is the pressure of the flask
Step-by-step explanation:
Combined gas law defines the relationship of pressure, absolute temperature and volume of a gas under different conditions. The formula is:
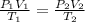
In the problem, initial conditions of the gas are:
517mmHg = P₁
24°C + 273.15 = 297.15K = T₁
95mL = V₁
And final conditions are:
225mL = V₂
8.0°C + 273.15 = 265.15K = T₂
Replacing:
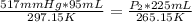
P₂ = 195mmHg is the pressure of the flask