Answer:
Equal to 0.253 mol.
Step-by-step explanation:
Hello,
In this case, since hydrogen and water are in a 2:2 molar relationship, the number of moles of hydrogen necessary to produce 0.253 mol of water will be also 0.253 mol as shown on the following stoichiometric factor:
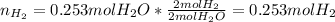
Therefore, The number of moles of hydrogen that is needed to produce 0.253 mol of water is equal to 0.253 mol.
Best regards.