Answer:
The change in temperature ΔT that occurs when 8000 J of energy (q) is used to heat up a mass (m) of 75 g of water is 25°C
Step-by-step explanation:
The formula for change in heat supplied to a body is given as follows;
ΔH = m·C·ΔT
Where:
ΔH = Heat supplied to the body = 8000J
m = Mass of the body = 75 g
ΔT =
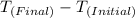 = Temperature change experienced by the body
= Temperature change experienced by the body
c = Specific heat capacity of water = 4.186 J/g
Therefore, 8000 J = 75 g × 4.186 J/(g·°C) × ΔT
Therefore;
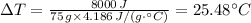
Hence the change in temperature ΔT that occurs when 8000 J of energy (q) is used to heat up a mass (m) of 75 g of water rounded to the nearest whole number = 25°C.