Answer:
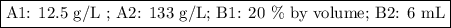
Step-by-step explanation:
A. Concentration
Question 1
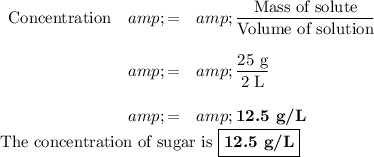
Question 2
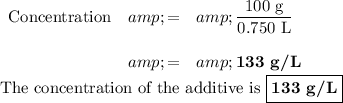
B. Percent by volume
Question 1
The formula for percent by volume is
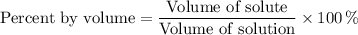
If your solution contains 50 mL of rubbing alcohol and 200 mL of water, the volume of solution is 250 mL,
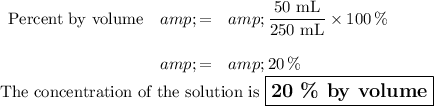
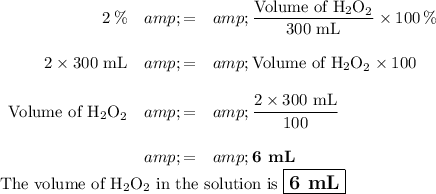
Question 2
If you have 300 mL of a solution that is 2 % v/v H₂O₂,