Answer:
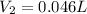
Step-by-step explanation:
Hello,
In this case, we apply the Boyle's law as an inversely proportional relationship allowing us to understand the pressure-volume behavior as shown below:

In such a way, solving for the final volume V2, we obtain:
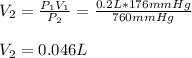
Best regards.