Stoichiometry and Solutions
When solving stoichiometry problems with solutions, there are some equations and concepts we must know:
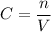 , where C = concentration, n = moles and V = volume
, where C = concentration, n = moles and V = volume
- With general stoichiometry, we always must write and balance a chemical equation. This can help us write mole ratios based on the coefficients of products/reactants.
Solving the Question
First, write and balance a chemical equation with NaOH and H2SO4:
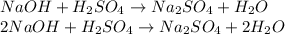
We're given:
Now, solve for moles of NaOH using a mole ratio:
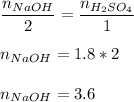
Therefore, the moles of NaOH is 3.6 moles.
Finally, solve for the volume of NaOH using
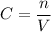 :
:
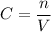
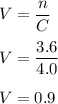
Therefore, the volume of NaOH is 0.9 L.
Rounded to significant figures, this would be 0.90 L.
Answer
0.90 L