Answer:
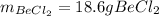
Step-by-step explanation:
Hello,
In this case, we first relate the given formula units of beryllium chloride as molecules with moles by using the Avogadro's number:
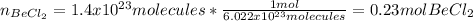
Now, we use its molar mass (80g/mol) to compute the required mass:
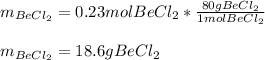
Best regards.