Answer:
The molarity is 0.546

Step-by-step explanation:
In a mixture, the chemical present in the greatest amount is called a solvent, while the other components are called solutes.
Molarity is a unit of concentration based on the volume of a solution and is defined as the number of moles of solute per liter of solution. In other words, molarity is the number of moles of solute that are dissolved in a given volume.
The Molarity of a solution is then expressed as:
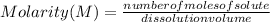
Molarity is expressed in units (
 ).
).
In this case:
- number of moles of solute: 35.5 mole
- dissolution volume: 65 L
Replacing:
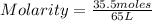
Solving:
Molarity=0.546

The molarity is 0.546
