Answer : The products of the acid-base reaction between
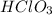 and LiOH are,
and LiOH are,
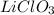 and
and

Explanation :
Neutralization reaction : It is a type of chemical reaction in which an acid react with a base to give salt and water as a product that means it reacts to give a neutral solution. It is also known as acid-base reaction.
The acid-base reaction will be:
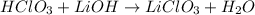
Therefore, the products of the acid-base reaction between
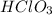 and LiOH are,
and LiOH are,
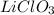 and
and
