Answer:
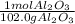
Step-by-step explanation:
Hello,
In this case, as the molar mass means that 1 mole of a substance has an specific amount of grams of the same substance, for aluminium oxide, we see that in one mole, there are 102.0 grams of it, which is symbolized by:
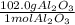
Nonetheless, the inverted molar mass, which is useful to compute the moles of aluminium oxide when mass is given is defined as:
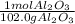
Which has now moles above and grams below.
Best regards.