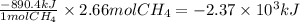Answer:
-2.37 × 10³ kJ
Step-by-step explanation:
Let's consider the following thermochemical equation.
CH₄(g) + 2 O₂(g) ⇒ CO₂(g) + 2 H₂O(l) ΔH = -890.4 kJ
Step 1: Calculate the moles corresponding to 42.6 g of methane
The molar mass of CH₄ is 16.04 g/mol. We will use this to calculate the moles corresponding to 42.6 g of CH₄.
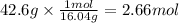
Step 2: Calculate the heat released from burning 2.66 moles of methane
According to the thermochemical equation, 890.4 kJ are released from burning 1 mol of methane. Then,