Answer: 1082 g of carbon dioxide are produced for every 350 grams of octane reacted in the engine
Step-by-step explanation:
To calculate the moles :
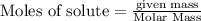
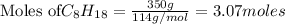
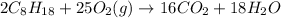
According to stoichiometry :
As 2 moles of octane give = 16 moles of

Thus 3.07 moles of octane will give =
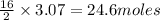 of
of

Mass of
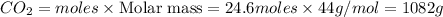
Thus 1082 g of carbon dioxide are produced for every 350 grams of octane reacted in the engine