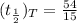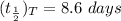Answer:
The effective half-life is
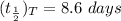
Step-by-step explanation:
From the question we are told that
The excretion half-life is
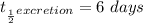
The radiation half-life is
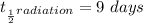
The decay due to excretion is mathematically represented as
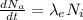
Where
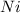 is the original number of tracers
is the original number of tracers
The decay due to excretion is mathematically represented as
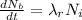
Now from the question we are total decay is as result of the combined decay of both processes
We have that
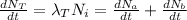
Substituting for the formula above
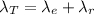
Generally the formula for half-life is
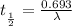
So
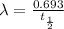
Substituting this into the above equation
![[(0.693)/(t_(1)/(2) ) ]_T =[(0.693)/(t_(1)/(2) ) ]_e + [(0.693)/(t_(1)/(2) ) ]_r](https://img.qammunity.org/2021/formulas/physics/college/hpwrsn2wzckryzkub9xjudpdcul2k5jy0l.png)
![[(1)/(t_(1)/(2) ) ]_T =([(1)/(t_(1)/(2) ) ]_e + [(1)/(t_(1)/(2) ) ]_r)/([(1)/(t_(1)/(2) ) ]_e * [(1)/(t_(1)/(2) ) ]_r)](https://img.qammunity.org/2021/formulas/physics/college/titerp924dsz2e9gvr6524pt5z5vvphweo.png)
Substituting values
![[(1)/(t_(1)/(2) ) ]_T =( 9+6)/(9 * 6)](https://img.qammunity.org/2021/formulas/physics/college/ms8klvfwgrvef4a1fc5ggb30w8c9n30ax3.png)
![[(1)/(t_(1)/(2) ) ]_T =( 15)/(54)](https://img.qammunity.org/2021/formulas/physics/college/d0ffnfuwfew7isqaj588w8wemnu4ywmyvs.png)