Answer : The correct option is (d) 14.6 %
Explanation :
Percent composition : It is calculated by dividing the mass of each element in one mole of the compound that means dividing the mass of each element by the total molar mass of the compound.
Formula used :
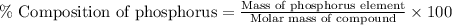
Molar mass of compound,
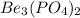 = 216.9 g/mol
= 216.9 g/mol
Molar mass of phosphorus = 30.9 g/mol
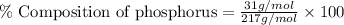
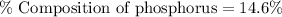
Therefore, the percent composition of phosphorus in
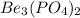 is 14.6 %.
is 14.6 %.