Answer:
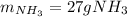
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
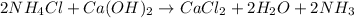
Hence, since two different amount of reactants are reacting one must identify the limiting reactant before computing the yield of ammonia. For that reason, we compute the available moles of ammonium chloride and the moles of ammonium chloride that will be consumed by 130 g of calcium hydroxide as shown below:
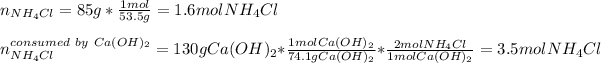
For that reason, the ammonium chloride is the limiting reactant (less available moles), thus, the yielded grams of ammonia turn out:
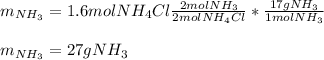
Best regards.