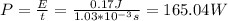Answer:
a) 9*10^18 photons
b) 165.04 W
Step-by-step explanation:
a. To find the number of photons you first calculate the energy used to rise the temperature of the flesh ans the energy necessary to evaporate the water. All this contribution is given by the following formula:
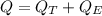
QT: energy to rise the temperature
QE: energy to evaporate the water
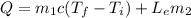 ( 1 )
( 1 )
m1: mass of the flesh
m2: mass of the water
Le: heat of vaporization of water
You take into account that approximately 75% of the substance in flesh is water. Hence, the mass of water will be:
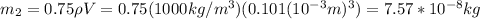
And the mass of flesh:
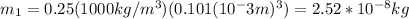
you replace the values of the parameters in the equation (1) to obtain:
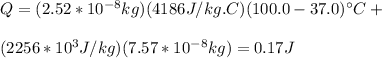 (2)
(2)
0.17 J is the energy required
Next, you calculate the energy of a 10.6µm photon:
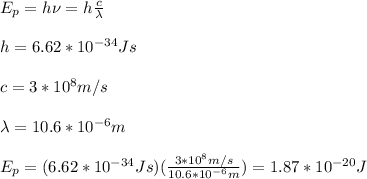
you divide the energy obtained in (2) with Ep to get the number of photons:
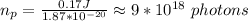
b. To find the minium power output you use: