Answer:
The new pressure is 44.4 kPa.
Step-by-step explanation:
We have,
Initial volume,
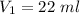
Initial pressure,
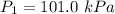
It is required to find the new pressure when the volume is increased to 50 ml. The relationship between pressure and volume is known as Boyle's law.
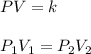
 is final pressure
is final pressure
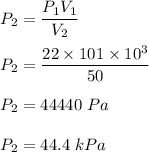
So, new pressure is 44.4 kPa.