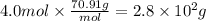Answer:
2.8 × 10² g
Step-by-step explanation:
Given data
Step 1: Convert 25°C to the absolute scale
When working with gases, we have to convert the temperatures to the Kelvin scale, using the following expression.
K = °C + 273.15 = 25°C + 273.15 = 298 K
Step 2: Calculate the moles of chlorine gas
We will use the ideal gas equation.
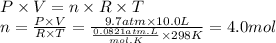
Step 3: Calculate the mass of chlorine gas
The molar mass of Cl₂ is 70.91 g/mol. Then,