Answer:
the molecular formula is C₁₄H₈O₂
Step-by-step explanation:
Given that
The freezing point of camphor is lowered by 22.3°C
Amount of anthraquinone is 1.32g
depression constant is 40°C.kg.mol
Use the freezing-point depression equation
Molecular weight of solute, m =
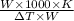
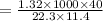
m = 208g/mol
empirical formula of Anthraquinone is C7H4O
empirical formula weight = 104 g/mol
m = Molecular formula weight / empirical formula weight
m = 208 / 104
208.2 / 104.1 = 2
molecular formula = (C₇H₄O)₂
so, the molecular formula is C₁₄H₈O₂