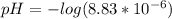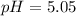Answer:
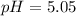
Step-by-step explanation:
pH is derived from the concentration of hydronium ions in a solution. Hydrocyanic acid is HCN.
First, we shall figure out the moles of HCN:
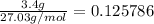
If HCN was a strong acid:
HCN has a 1:1 ratio of H+ ions, the moles of H+ is also the same.
To find the molarity, we now divide by Liters. This gets us:
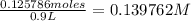
Finally, we plug it into the definition of pH:
![pH = -log[H^(+) ]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/ar3492znyc9nzspcx4dnt82g0fae6ndb4m.png)
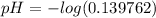
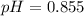
However, since HCN is a weak acid, it only partially dissociates. The
 of HCN is
of HCN is
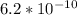 .
.
![K_a = ([H^+][A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/middle-school/nld1sr47d7pxk8jlk7fisltuqq4j9nmfkl.png)
We can use an ice table to determine that when x = H+,
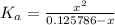
![[H^+] = 8.83*10^(-6)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/cd9bhsbhm3kovv67j8066xbz6aj8pi5d9r.png)
![pH = -log[H^(+) ]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/ar3492znyc9nzspcx4dnt82g0fae6ndb4m.png)